You are here
Fauci sees decision on US resuming J&J vaccine by Friday
By AFP - Apr 19,2021 - Last updated at Apr 19,2021
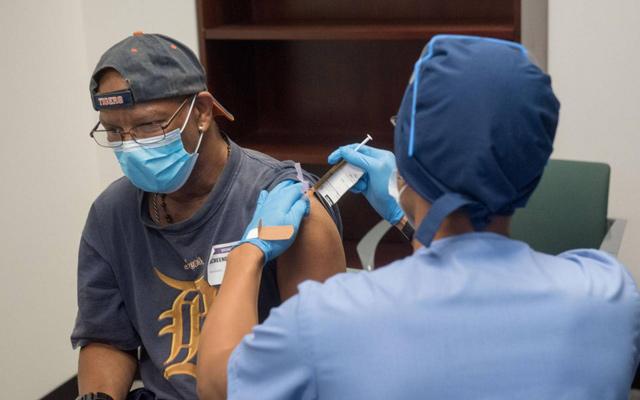
In this image courtesy of the Henry Ford Health System, a volunteer is given the Moderna vaccine on August 5, 2020, in Detroit, Michigan (AFP photo)
WASHINGTON — A decision on whether to end a US pause in vaccinations with the Johnson & Johnson COVID-19 shot is likely by Friday, top US pandemic adviser Anthony Fauci said Sunday.
A government-convened expert panel has been assessing the vaccine's possible links to a clotting disorder seen in a half-dozen relatively young women, none with previously known clotting disorders.
Meantime, the European Medicines Agency (EMA) said on Friday it expected to rule on the safety of the Johnson & Johnson's shot on Tuesday after evaluating data on blood clotting.
Fauci, in an interview on ABC's "This Week", said that by Friday "we should have an answer as to where we're going with it. I would think that we're not going to go beyond Friday in the extension of this pause".
While saying he did not want to get ahead of the expert panel convened by the Centres for Disease Control and Prevention (CDC), Fauci noted that the clotting disorder was "an extraordinarily rare event".
"I believe we'll get back with it," he said, though possibly with some restrictions or warnings on its use.
Vulnerable groups
US health authorities have reported six cases of women developing brain clots along with low blood platelet counts, including one death, within two weeks of getting the one-dose vaccine. All the women were between 18 and 49.
The shot has been given to some 7.2 million Americans.
Some experts have opposed continuing the pause, fearing it might disproportionately impact vulnerable groups that are easier to reach through a single-dose vaccine that can be stored in fridges.
But most felt that given the highly serious nature of the clots, and given the abundance in the United States of other vaccines that do not have the same safety concern, further study was necessary.
CDC Director Rochelle Walensky has said that the symptoms in the women suffering clotting disorders were consistent with rare side effects from the AstraZeneca vaccine seen in Europe.
Some European officials have hinted that the European Union might not order the AstraZeneca shot again after questions about side effects and delays in its delivery.
Both the J&J and the AstraZeneca vaccines are based on adenovirus vector technology, as are Russia’s Sputnik V and China’s CanSino.
The clotting problem has not been linked to the Pfizer or Moderna vaccines.
US officials have emphasised that they have ordered more than enough supply of the Moderna and Pfizer vaccines to cover the adult population by the end of July.
While the US has become a world leader in vaccinations, Fauci said the country remained in a “precarious position”, with too many people ignoring health precautions.
“We’re having a seven-day average of over 60,000 new infections per day. That’s a place you don’t want to be,” he said.
Some 30 million people are being vaccinated every week in the US.
But Fauci warned: “We also have to make sure that people don’t throw caution to the wind and declare victory prematurely. That’s not the time to do that.”
Related Articles
BRUSSELS — The EU said on Wednesday it would get 50 million Pfizer vaccine doses earlier than expected, as rival drugmaker AstraZeneca faced
BRUSSELS — A co-founder of the global scheme to provide vaccines for poor people said onThursday that India was delaying exports of much-ne
GENEVA — The EU's medicines regulator said onTuesday there was so far "no indication" the AstraZeneca vaccine causes blood clots, urging cou